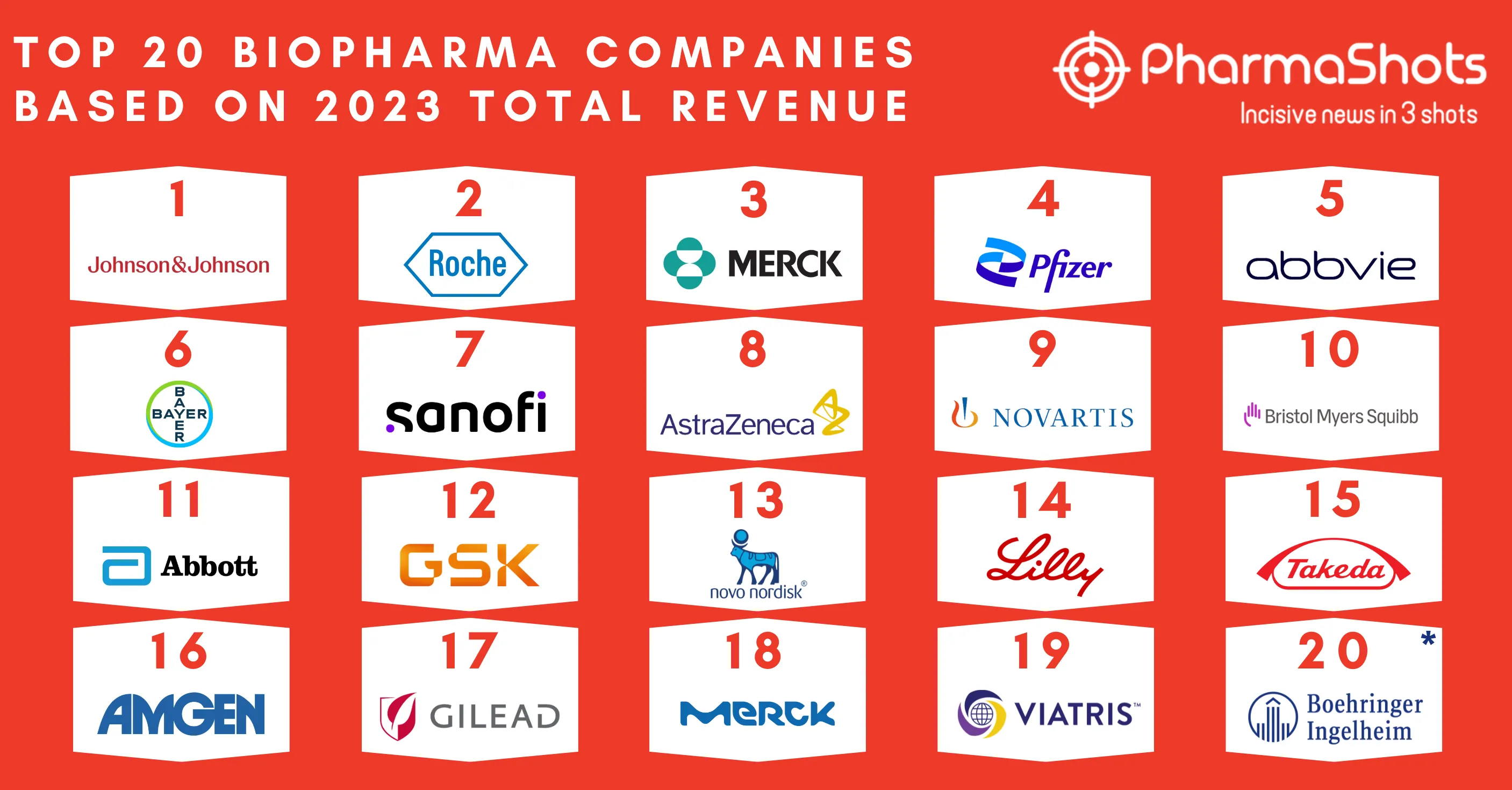
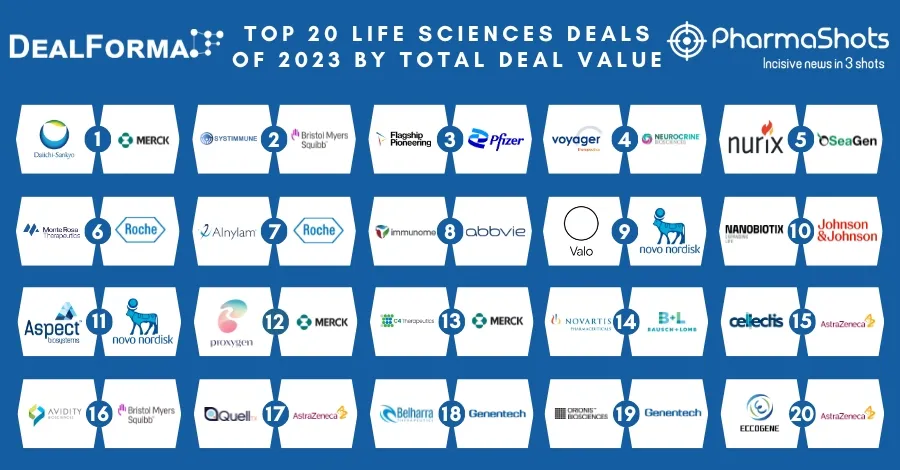
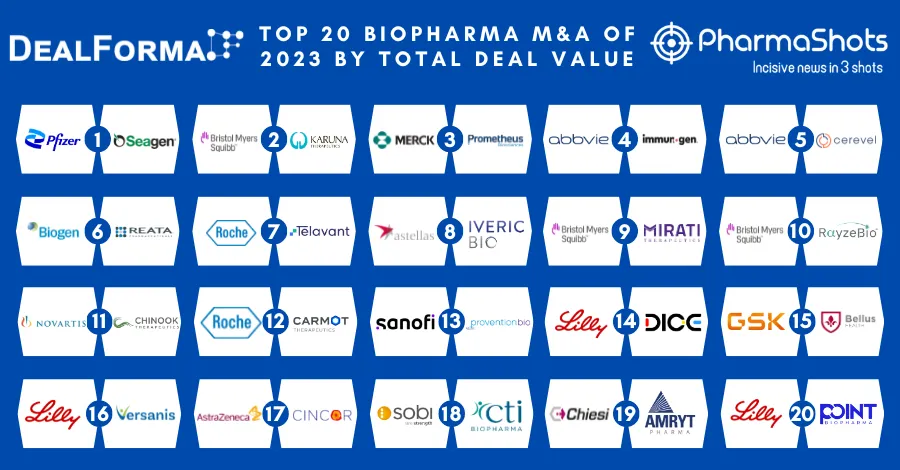
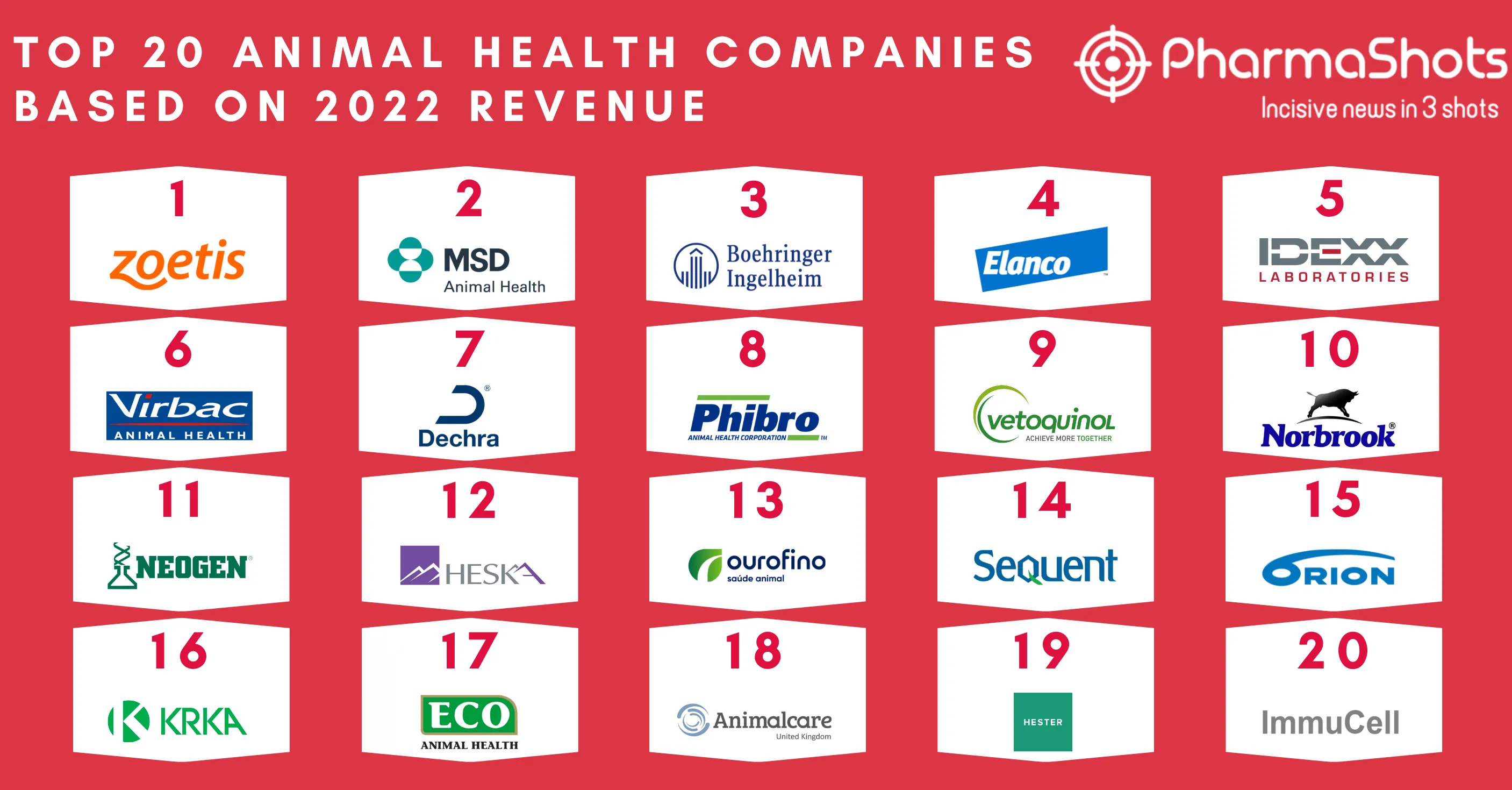
AstraZeneca & Merck Reports P-III SOLO-1 Trial Results of Lynparza (olaparib) for 1L+ Treatment in Advance BRCAm Ovarian Cancer at ESMO18
Shots:
- The P-III SOLO-1 Trial includes assessing of Lynparza (olaparib) (300mg bid) vs PBO in 391 patients in ratio (2:1) with newly-diagnosed- BRCA1/2 mutation who were in CR or PR following 1L Pt-based CT
- P-III SOLO-1 Trial results: reduction in risk of disease progression or death by 70%; PFS @ 36 mos. (60% vs 27%); mPFS for Lynparza didn’t reached 13.8 months as for PBO
- Lynparza (olaparib) is a novel PARPi- used in 20-000 patients worldwide and is approved for advanced OC and mBC. In July 2017- AZ & Merck globally collaborated to co-develop and co-commercialise Lynparza
Ref: Astrazeneca | Image: Astrazeneca
Click here to read the full press release

This content piece was prepared by our former Senior Editor. She had expertise in life science research and was an avid reader. For any query reach out to us at connect@pharmashots.com